
Back ذرة الهيدروجين Arabic Atom hidrogén BAN Водороден атом Bulgarian Wasserstoffatom German Átomo de hidrógeno Spanish اتم هیدروژن Persian Atome d'hydrogène French 氕 GAN אטום המימן HE हाइड्रोजन परमाणु Hindi
 | |
| General | |
|---|---|
| Symbol | 1H |
| Names | hydrogen atom, 1H, H-1, protium |
| Protons (Z) | 1 |
| Neutrons (N) | 0 |
| Nuclide data | |
| Natural abundance | 99.985% |
| Half-life (t1/2) | stable |
| Isotope mass | 1.007825 Da |
| Spin | 1/2 |
| Excess energy | 7288.969±0.001 keV |
| Binding energy | 0.000±0.0000 keV |
| Isotopes of hydrogen Complete table of nuclides | |
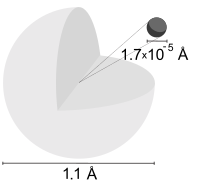
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral hydrogen atom contains a nucleus of a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe.[1]
In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, a hydrogen atom tends to combine with other atoms in compounds, or with another hydrogen atom to form ordinary (diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).
Atomic spectroscopy shows that there is a discrete infinite set of states in which a hydrogen (or any) atom can exist, contrary to the predictions of classical physics. Attempts to develop a theoretical understanding of the states of the hydrogen atom have been important to the history of quantum mechanics, since all other atoms can be roughly understood by knowing in detail about this simplest atomic structure.
- ^ Palmer, D. (13 September 1997). "Hydrogen in the Universe". NASA. Archived from the original on 29 October 2014. Retrieved 23 February 2017.